Clipping Board » Drug Poisoning ─ It is necessary to be aware of the toxic side effects before taking medication.

 2010/01/28 [United Evening News / Reporters Wang Huilun, Li Shuren, Lin Jinxiu]
2010/01/28 [United Evening News / Reporters Wang Huilun, Li Shuren, Lin Jinxiu]
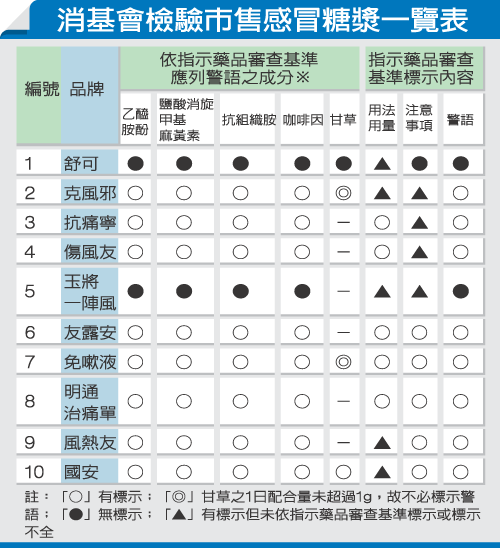
The habit of Taiwanese people arbitrarily consuming cough syrup when feeling unwell from a cold is severe. The Consumers' Foundation (CF) released a survey result this morning, revealing that out of 10 cough syrup products tested from the market, 90% had warning labels printed on the inner packaging, making them nearly invisible to consumers. Long-term and excessive consumption can easily lead to liver and kidney failure, hypokalemia, and other harms. CF Chairman Xie Tianren stated: "The high rates of kidney failure and dialysis in Taiwan are largely due to the excessive consumption of cough syrup. The authorities should recognize the severity of this issue and strengthen regulations and inspections."
According to statistics from an international medical service company, Taiwan’s annual sales of cough syrup amount to approximately NT$1.5 billion. Assuming each bottle costs NT$20, this means Taiwanese people consume at least 210,000 bottles per day.
CF Secretary-General Huang Yusheng said: "Cough syrup is an over-the-counter medication, and the recommended dosage is only 10c.c. per use. However, some consumers drink an entire bottle at once or even take it three times a day before bed. This is because advertisements and packaging often fail to include warnings, ingredients, or information about the side effects of excessive consumption. People mistakenly believe that because it tastes sweet, it’s both pleasant and curative. In reality, cough syrup contains acetaminophen, antihistamines, and caffeine. Overconsumption can lead to addiction and even severe consequences like kidney dialysis."
Currently, under Article 75 of the Pharmaceutical Affairs Act, cough syrup is classified as an over-the-counter medication and must include usage warnings and other precautions.
The CF’s inspection found that "Shuke Cough Syrup" failed to label side effects, contraindications, and precautions; "Kefengxie Cough Syrup" and "Kangtongning Cough Syrup" did not include batch numbers; and "Yujiang Yizhenfeng" omitted side effect warnings. These violations will incur fines ranging from NT$30,000 to NT$150,000 under the Pharmaceutical Affairs Act.
Additionally, while 90% of cough syrups did include warnings, they were almost uniformly printed on the inner packaging, making them practically invisible. Xie Tianren remarked: "If they’re called warnings, they should be clearly visible. Some advertisements even promote slogans like 'Drinking for 30 years and still healthy,' making it seem like the syrup poses no risks at all. If you’re truly sick, you should see a doctor."
Dr. Zhao Kai from Hsinchu Cathay General Hospital’s Emergency Department also noted that Taiwan’s uneven distribution of medical resources means that farmers, fishermen, and workers in mountainous, rural, or coastal areas often rely on pharmacists for medication advice when they fall ill. If cough syrup warnings are unclear, it can lead to long-term overconsumption. Moreover, patients with chronic conditions like heart disease, hypertension, or diabetes who take long-term medications may experience dangerous interactions, such as hyperglycemia, drowsiness, or mental confusion, which could lead to accidents. Therefore, these three high-risk groups should avoid consuming cough syrup altogether.
Department of Health: Will Evaluate Warning Labels on Over-the-Counter Drugs
In response to the CF’s criticism, the Department of Health (DOH) stated at noon that it will commission scholars this year to evaluate warning labels on over-the-counter drugs. The DOH will gather the latest regulations on warnings and precautions for cold remedies from advanced countries like the U.S., Europe, and Japan, and reassess the labeling of cold remedies on the market to ensure consumer safety.
Dai Xueyong, Section Chief of the DOH’s Food and Drug Administration’s Drug Division, explained that under current regulations, products classified as "over-the-counter cold remedies" must include item names, warnings, and precautions. However, due to limited space on outer packaging, manufacturers may choose to display only one of these.
Dai Xueyong emphasized that, according to the Pharmaceutical Affairs Act, manufacturers can decide whether to print warnings on the drug label, package insert, or outer box based on circumstances.If consumers have concerns about product usage before purchasing, they can proactively consult medical professionals to obtain complete medication information.
Taipei City Health Department: Conducts irregular spot checks
Regarding the Consumer Foundation's findings that most over-the-counter cough syrup warnings are placed inside the box, making them difficult for the public to notice, Chiang Yu-mei, Director of the Taipei City Health Department's Food and Drug Division, advised that consumers should practice self-management and carefully review the information when purchasing and taking such medications.
The Taipei City Health Department does not specifically inspect whether warnings are appropriately placed but will conduct irregular spot checks on over-the-counter medications with unauthorized packaging changes or abnormal drug quality to ensure public medication safety.
Source: http:/ / mag. udn. com/ mag/ life/ ……_SUB_ID=1414& f_ART_ID=233271
Cold Syrup Overuse Leads to Kidney Failure


The habit of Taiwanese people arbitrarily consuming cough syrup when feeling unwell from a cold is severe. The Consumers' Foundation (CF) released a survey result this morning, revealing that out of 10 cough syrup products tested from the market, 90% had warning labels printed on the inner packaging, making them nearly invisible to consumers. Long-term and excessive consumption can easily lead to liver and kidney failure, hypokalemia, and other harms. CF Chairman Xie Tianren stated: "The high rates of kidney failure and dialysis in Taiwan are largely due to the excessive consumption of cough syrup. The authorities should recognize the severity of this issue and strengthen regulations and inspections."
According to statistics from an international medical service company, Taiwan’s annual sales of cough syrup amount to approximately NT$1.5 billion. Assuming each bottle costs NT$20, this means Taiwanese people consume at least 210,000 bottles per day.
CF Secretary-General Huang Yusheng said: "Cough syrup is an over-the-counter medication, and the recommended dosage is only 10c.c. per use. However, some consumers drink an entire bottle at once or even take it three times a day before bed. This is because advertisements and packaging often fail to include warnings, ingredients, or information about the side effects of excessive consumption. People mistakenly believe that because it tastes sweet, it’s both pleasant and curative. In reality, cough syrup contains acetaminophen, antihistamines, and caffeine. Overconsumption can lead to addiction and even severe consequences like kidney dialysis."
Currently, under Article 75 of the Pharmaceutical Affairs Act, cough syrup is classified as an over-the-counter medication and must include usage warnings and other precautions.
The CF’s inspection found that "Shuke Cough Syrup" failed to label side effects, contraindications, and precautions; "Kefengxie Cough Syrup" and "Kangtongning Cough Syrup" did not include batch numbers; and "Yujiang Yizhenfeng" omitted side effect warnings. These violations will incur fines ranging from NT$30,000 to NT$150,000 under the Pharmaceutical Affairs Act.
Additionally, while 90% of cough syrups did include warnings, they were almost uniformly printed on the inner packaging, making them practically invisible. Xie Tianren remarked: "If they’re called warnings, they should be clearly visible. Some advertisements even promote slogans like 'Drinking for 30 years and still healthy,' making it seem like the syrup poses no risks at all. If you’re truly sick, you should see a doctor."
Dr. Zhao Kai from Hsinchu Cathay General Hospital’s Emergency Department also noted that Taiwan’s uneven distribution of medical resources means that farmers, fishermen, and workers in mountainous, rural, or coastal areas often rely on pharmacists for medication advice when they fall ill. If cough syrup warnings are unclear, it can lead to long-term overconsumption. Moreover, patients with chronic conditions like heart disease, hypertension, or diabetes who take long-term medications may experience dangerous interactions, such as hyperglycemia, drowsiness, or mental confusion, which could lead to accidents. Therefore, these three high-risk groups should avoid consuming cough syrup altogether.

Department of Health: Will Evaluate Warning Labels on Over-the-Counter Drugs
In response to the CF’s criticism, the Department of Health (DOH) stated at noon that it will commission scholars this year to evaluate warning labels on over-the-counter drugs. The DOH will gather the latest regulations on warnings and precautions for cold remedies from advanced countries like the U.S., Europe, and Japan, and reassess the labeling of cold remedies on the market to ensure consumer safety.
Dai Xueyong, Section Chief of the DOH’s Food and Drug Administration’s Drug Division, explained that under current regulations, products classified as "over-the-counter cold remedies" must include item names, warnings, and precautions. However, due to limited space on outer packaging, manufacturers may choose to display only one of these.
Dai Xueyong emphasized that, according to the Pharmaceutical Affairs Act, manufacturers can decide whether to print warnings on the drug label, package insert, or outer box based on circumstances.If consumers have concerns about product usage before purchasing, they can proactively consult medical professionals to obtain complete medication information.
Taipei City Health Department: Conducts irregular spot checks
Regarding the Consumer Foundation's findings that most over-the-counter cough syrup warnings are placed inside the box, making them difficult for the public to notice, Chiang Yu-mei, Director of the Taipei City Health Department's Food and Drug Division, advised that consumers should practice self-management and carefully review the information when purchasing and taking such medications.
The Taipei City Health Department does not specifically inspect whether warnings are appropriately placed but will conduct irregular spot checks on over-the-counter medications with unauthorized packaging changes or abnormal drug quality to ensure public medication safety.
Source: http:/ / mag. udn. com/ mag/ life/ ……_SUB_ID=1414& f_ART_ID=233271

