Clipping Board » Illness Enters through Mouth ─ The information that has been made public is often just the tip of the iceberg...

 April 10, 2017
April 10, 2017
On March 15, the Taiwan Food and Drug Administration (TFDA) under the Ministry of Health and Welfare officially announced amendments to the "Pesticide Residue Tolerance Standards," revising and adding 128 pesticide residue limits for 22 types of pesticides in fruits, vegetables, and other agricultural products. Additionally, on March 13, the TFDA proposed 351 pesticide residue limits for agricultural products, which will be officially announced in May after a 60-day review period.
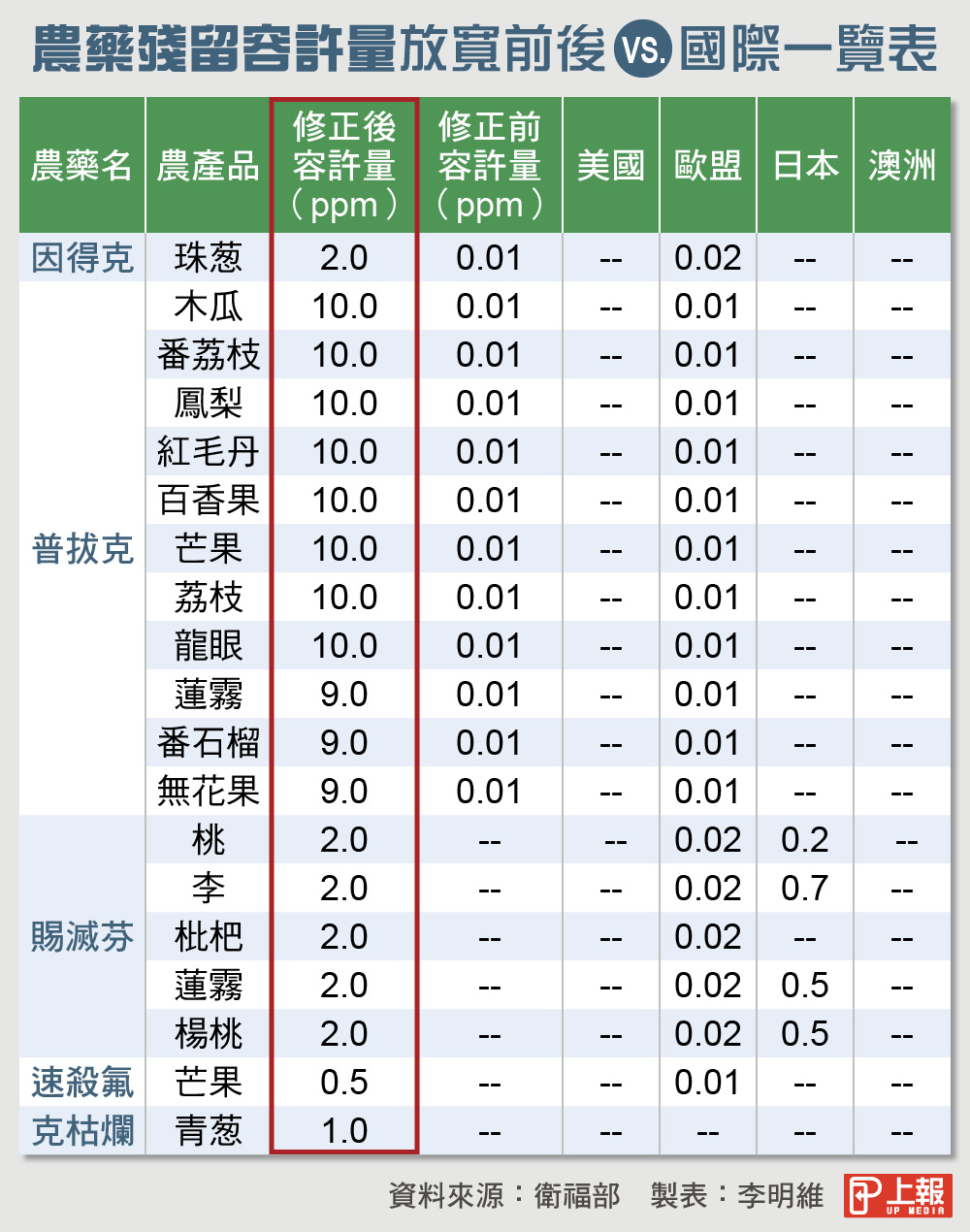
Among the officially announced revisions on March 15, eight commonly consumed fruits—including papaya, sugar apple, pineapple, rambutan, passion fruit, mango, lychee, and longan—saw a significant relaxation in the tolerance for the pesticide "propargite." The limit was raised from the original 0.01 ppm (aligned with the EU standard) to 10 ppm—a 1,000-fold increase. For three other fruits—wax apple, guava, and fig—the tolerance for propargite was raised from 0.01 ppm to 9 ppm, a 900-fold increase. However, in the U.S., Japan, and Australia, propargite is not permitted in these fruits at all. The Ministry of Health and Welfare apparently believes that Taiwanese people can tolerate much higher pesticide residues than those in Europe, the U.S., and Japan.
The 128 pesticide residue limits for 22 types of pesticides in agricultural products were first proposed in December of the previous year. Despite objections from advisory committee members, the TFDA proceeded with the revisions as submitted by the Council of Agriculture (COA) and officially announced them on March 15. When questioned by legislators on March 26, TFDA Director-General Wu Hsiu-mei even stated that pesticide regulation would adopt a "single-whip" approach, meaning the COA’s Bureau of Animal and Plant Health Inspection and Quarantine would oversee pesticide applications, reviews, approvals, and residue standards—a clear dereliction of duty by the TFDA.
According to the National Institute of Environmental Health Sciences’ Food Safety Information Network, propargite is an insecticide that can cause gastrointestinal discomfort if ingested. In animal testing, beagles exposed to propargite for two years developed erosive gastritis. The World Health Organization recommends a maximum daily intake of 0.1 mg per kilogram of body weight for propargite.
While public attention has focused on the government’s relaxation of standards for pesticides like "fluopyram" (banned in Japan, Australia, and the EU but now permitted in tea) and "dimethomorph" (with increased tolerance in lettuce), statistics from Legislator Lin Shu-fen’s office show that the two announcements collectively added 451 new pesticide residue limits, relaxed 8 existing ones, restricted only 19, and canceled just 1. Moreover, 46 of these limits are "uniquely high" compared to international standards.
In the March 15 announcement, multiple pesticide residue limits for fruits and vegetables were significantly raised. Aside from the drastic increase in propargite tolerance for eight fruits (10 ppm, 1,000 times higher than the EU standard) and three others (9 ppm, 900 times higher), the U.S., Japan, and Australia prohibit any detectable levels of propargite in these fruits.
Another pesticide, "simeconazole," saw its tolerance raised from "not detectable" to 2 ppm for peaches, plums, loquats, wax apples, guavas, and carambolas—far exceeding the EU’s 0.02 ppm and Japan’s 0.2–0.7 ppm limits.
Similarly, the tolerance for the pesticide "sulfoxaflor" in mangoes was raised from "not detectable" (the standard in Taiwan, the U.S., Japan, and Australia) to 0.5 ppm, despite the EU’s limit being only 0.01 ppm.Additionally, the permissible residue level of the newly added pesticide "Kekulan" on green onions, an essential ingredient in Taiwanese cooking, has been raised from the previous standard of "not detectable" (aligned with the EU, U.S., Japan, and Australia) to an allowable 1 ppm.
The insecticide "Simifen" has been experimentally confirmed to potentially cause uterine or testicular cancer, while the insecticide "Sushafu" is opposed by international environmental groups due to findings that it accelerates the decline of bee populations.
In reality, the 128 revised permissible residue levels for 22 pesticides on fruits and vegetables, officially announced on the 15th, were first proposed as early as December 13 last year. At that time, the Food and Drug Administration (FDA) under the Ministry of Health and Welfare convened the "Food Safety and Nutrition Advisory Committee" to review the proposed revisions submitted by the Bureau of Animal and Plant Health Inspection and Quarantine (BAPHIQ) under the Council of Agriculture. However, the FDA only conducted a "documentary review." During the advisory process, some committee members argued that the permissible residue levels for several pesticides, including "Pubake," "Simifen," "Sushafu," "Deli," and "Kekulan," should be significantly lowered to align more closely with EU standards rather than being set much higher.
Some advisory committee members also questioned why many items in the revised list—which originally had no existing permissible levels—were set higher than "international standards," such as "Yindeke," "Pubake," "Simifen," "Sushafu," and "Sanfumin." They demanded that the Ministry of Health and Welfare explain the "necessity" and "rationality" behind these standards to "prevent consumer groups from raising concerns about the permissible residue levels."
However, the FDA responded entirely from the perspective of the Council of Agriculture, seemingly forgetting its role as the regulatory authority responsible for food safety.
The FDA avoided addressing why many standards were set higher than "international levels," merely stating that these permissible residue levels were "continuously adjusted" in accordance with the Council of Agriculture's pesticide registration and usage guidelines, as well as field trial residue data or monitoring results. The FDA claimed it was "necessary" to establish reasonable residue limits to "prevent farmers from exceeding permissible levels even when following recommended usage methods."
The FDA even confidently explained to the advisory committee that the revised pesticide residue levels would result in estimated total intake below 80% of the ADI (Acceptable Daily Intake), claiming they were "within safe limits."
However, some committee members pointed out that the estimated intake for several pesticides in the list was "relatively high," approaching 80% of the ADI. Examples included "Dateman," "Fukesha," "Gushacao," "Yindeke," "Baikemin," and "Saisuan," all exceeding 70% of the ADI, with many nearing the upper daily intake limit. Since these intake estimates were based on adult standards, some committee members suggested the FDA also conduct intake assessments for children to ensure the protective adequacy of the residue limits.
The list of revised pesticide residue levels, announced on March 13, was also reviewed by the "Food Safety and Nutrition Advisory Committee" prior to its release. Again, committee members called for lowering permissible residue levels for many agricultural products. However, the FDA reiterated its stance from the Council of Agriculture's perspective, stating that "while some standards differ internationally, BAPHIQ has evaluated and established usage methods to provide reasonable pesticide options domestically." The FDA argued that setting appropriate residue limits was a necessary regulatory measure to prevent farmers from violating standards even when following recommended practices.
Source: http://www. upmedia. mg/ news_info. php? SerialNo=15135
MOHW Ignores Food Safety: Pesticide Residues in 8 Fruits Like Papaya and Pineapple Can Exceed EU Limits by 1,000 Times


On March 15, the Taiwan Food and Drug Administration (TFDA) under the Ministry of Health and Welfare officially announced amendments to the "Pesticide Residue Tolerance Standards," revising and adding 128 pesticide residue limits for 22 types of pesticides in fruits, vegetables, and other agricultural products. Additionally, on March 13, the TFDA proposed 351 pesticide residue limits for agricultural products, which will be officially announced in May after a 60-day review period.
Among the officially announced revisions on March 15, eight commonly consumed fruits—including papaya, sugar apple, pineapple, rambutan, passion fruit, mango, lychee, and longan—saw a significant relaxation in the tolerance for the pesticide "propargite." The limit was raised from the original 0.01 ppm (aligned with the EU standard) to 10 ppm—a 1,000-fold increase. For three other fruits—wax apple, guava, and fig—the tolerance for propargite was raised from 0.01 ppm to 9 ppm, a 900-fold increase. However, in the U.S., Japan, and Australia, propargite is not permitted in these fruits at all. The Ministry of Health and Welfare apparently believes that Taiwanese people can tolerate much higher pesticide residues than those in Europe, the U.S., and Japan.
The 128 pesticide residue limits for 22 types of pesticides in agricultural products were first proposed in December of the previous year. Despite objections from advisory committee members, the TFDA proceeded with the revisions as submitted by the Council of Agriculture (COA) and officially announced them on March 15. When questioned by legislators on March 26, TFDA Director-General Wu Hsiu-mei even stated that pesticide regulation would adopt a "single-whip" approach, meaning the COA’s Bureau of Animal and Plant Health Inspection and Quarantine would oversee pesticide applications, reviews, approvals, and residue standards—a clear dereliction of duty by the TFDA.
According to the National Institute of Environmental Health Sciences’ Food Safety Information Network, propargite is an insecticide that can cause gastrointestinal discomfort if ingested. In animal testing, beagles exposed to propargite for two years developed erosive gastritis. The World Health Organization recommends a maximum daily intake of 0.1 mg per kilogram of body weight for propargite.
While public attention has focused on the government’s relaxation of standards for pesticides like "fluopyram" (banned in Japan, Australia, and the EU but now permitted in tea) and "dimethomorph" (with increased tolerance in lettuce), statistics from Legislator Lin Shu-fen’s office show that the two announcements collectively added 451 new pesticide residue limits, relaxed 8 existing ones, restricted only 19, and canceled just 1. Moreover, 46 of these limits are "uniquely high" compared to international standards.
In the March 15 announcement, multiple pesticide residue limits for fruits and vegetables were significantly raised. Aside from the drastic increase in propargite tolerance for eight fruits (10 ppm, 1,000 times higher than the EU standard) and three others (9 ppm, 900 times higher), the U.S., Japan, and Australia prohibit any detectable levels of propargite in these fruits.
Another pesticide, "simeconazole," saw its tolerance raised from "not detectable" to 2 ppm for peaches, plums, loquats, wax apples, guavas, and carambolas—far exceeding the EU’s 0.02 ppm and Japan’s 0.2–0.7 ppm limits.
Similarly, the tolerance for the pesticide "sulfoxaflor" in mangoes was raised from "not detectable" (the standard in Taiwan, the U.S., Japan, and Australia) to 0.5 ppm, despite the EU’s limit being only 0.01 ppm.Additionally, the permissible residue level of the newly added pesticide "Kekulan" on green onions, an essential ingredient in Taiwanese cooking, has been raised from the previous standard of "not detectable" (aligned with the EU, U.S., Japan, and Australia) to an allowable 1 ppm.
The insecticide "Simifen" has been experimentally confirmed to potentially cause uterine or testicular cancer, while the insecticide "Sushafu" is opposed by international environmental groups due to findings that it accelerates the decline of bee populations.
In reality, the 128 revised permissible residue levels for 22 pesticides on fruits and vegetables, officially announced on the 15th, were first proposed as early as December 13 last year. At that time, the Food and Drug Administration (FDA) under the Ministry of Health and Welfare convened the "Food Safety and Nutrition Advisory Committee" to review the proposed revisions submitted by the Bureau of Animal and Plant Health Inspection and Quarantine (BAPHIQ) under the Council of Agriculture. However, the FDA only conducted a "documentary review." During the advisory process, some committee members argued that the permissible residue levels for several pesticides, including "Pubake," "Simifen," "Sushafu," "Deli," and "Kekulan," should be significantly lowered to align more closely with EU standards rather than being set much higher.

Some advisory committee members also questioned why many items in the revised list—which originally had no existing permissible levels—were set higher than "international standards," such as "Yindeke," "Pubake," "Simifen," "Sushafu," and "Sanfumin." They demanded that the Ministry of Health and Welfare explain the "necessity" and "rationality" behind these standards to "prevent consumer groups from raising concerns about the permissible residue levels."
However, the FDA responded entirely from the perspective of the Council of Agriculture, seemingly forgetting its role as the regulatory authority responsible for food safety.
The FDA avoided addressing why many standards were set higher than "international levels," merely stating that these permissible residue levels were "continuously adjusted" in accordance with the Council of Agriculture's pesticide registration and usage guidelines, as well as field trial residue data or monitoring results. The FDA claimed it was "necessary" to establish reasonable residue limits to "prevent farmers from exceeding permissible levels even when following recommended usage methods."
The FDA even confidently explained to the advisory committee that the revised pesticide residue levels would result in estimated total intake below 80% of the ADI (Acceptable Daily Intake), claiming they were "within safe limits."
However, some committee members pointed out that the estimated intake for several pesticides in the list was "relatively high," approaching 80% of the ADI. Examples included "Dateman," "Fukesha," "Gushacao," "Yindeke," "Baikemin," and "Saisuan," all exceeding 70% of the ADI, with many nearing the upper daily intake limit. Since these intake estimates were based on adult standards, some committee members suggested the FDA also conduct intake assessments for children to ensure the protective adequacy of the residue limits.
The list of revised pesticide residue levels, announced on March 13, was also reviewed by the "Food Safety and Nutrition Advisory Committee" prior to its release. Again, committee members called for lowering permissible residue levels for many agricultural products. However, the FDA reiterated its stance from the Council of Agriculture's perspective, stating that "while some standards differ internationally, BAPHIQ has evaluated and established usage methods to provide reasonable pesticide options domestically." The FDA argued that setting appropriate residue limits was a necessary regulatory measure to prevent farmers from violating standards even when following recommended practices.
Source: http://www. upmedia. mg/ news_info. php? SerialNo=15135

