Clipping Board » Research Report ─ The latest medical research reports and related news.

 2017-07-08 United Daily News | Oral account by Xu Zu'an, reported and compiled by journalist Wu Peirong
2017-07-08 United Daily News | Oral account by Xu Zu'an, reported and compiled by journalist Wu Peirong
Xu Zu'an: Researcher at the Institute of Biotechnology and Pharmaceutical Research, National Health Research Institutes
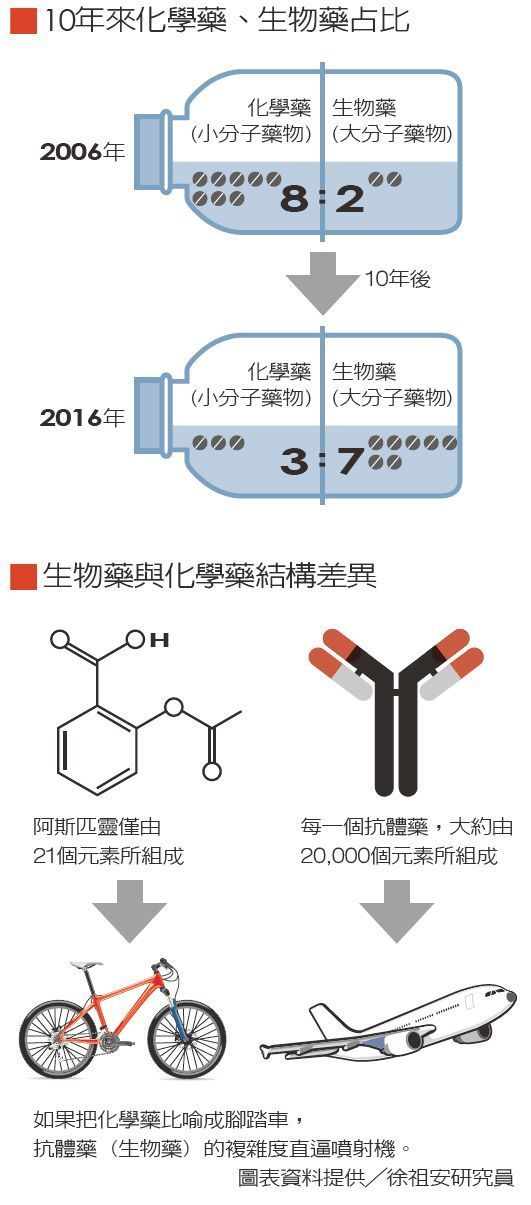
The global top 10 prescription drug rankings are quietly undergoing a transformation. In 2006, the list of top-selling prescription drugs was dominated by small-molecule drugs (chemical drugs), with only two large-molecule drugs (biologics) making it into the top 10. However, a decade later in 2016, the rankings saw a dramatic reversal, with biologics—especially antibodies and protein-based drugs—rising in popularity, while chemical drugs barely held onto the list, with only three remaining.
**High Toxicity and Unpredictability: Small-Molecule Drugs Hit Roadblocks**
Why has the market share of biologics and chemical drugs undergone such a drastic shift? Whether protein-based drugs or monoclonal antibodies, they are broadly categorized as large-molecule drugs, distinct from chemically synthesized drugs (small-molecule drugs) with clearly defined molecular structures. Over the past decade, large-molecule drugs have gone from niche to mainstream, and this shift can be examined from two perspectives.
From a research and development standpoint, small-molecule drugs (chemical drugs) tend to have higher toxicity. The most troublesome issue in small-molecule drug development is the unpredictable toxicological reactions that arise when the drug or its metabolites enter the human body, often leading to safety-related roadblocks and development failures. On the other hand, advancements in pharmaceutical technology have allowed large-molecule drugs—including hormones, proteins, and antibody-based drugs—to overcome production barriers, leading to continuous innovation and gradual dominance in the market.
**Bionic Replacement Over Confrontation: The Rise of Large-Molecule Drugs**
Another critical reason for the widespread clinical application of biologics is the shift in medical thinking. In the past, treatment approaches were confrontational, such as aggressively eliminating cancer cells. However, in recent years, researchers have reconsidered disease treatment through a "return to nature" mechanism. This "bionics" concept—mimicking the natural functioning of biological systems—has introduced new perspectives for diseases facing treatment bottlenecks. Biologics (large-molecule drugs) are developed by emulating the body's natural regulatory mechanisms, allowing them to overcome the limitations of traditional small-molecule drugs in targeting certain diseases while offering more stable efficacy and safety.
Take insulin, the earliest biologic, as an example. Insulin was discovered a century ago as a treatment for diabetes, but it wasn’t until recombinant protein technology matured that it could be mass-produced to benefit more patients. Before that, insulin extracted from pigs was used, which often triggered immune rejection in humans. Antibodies, a crucial part of the body’s defense system, have also become a hot topic in new drug development due to advancements in antibody production technology.
For diseases involving immune dysregulation, such as cancer and autoimmune disorders, biologics activate the body's natural regulatory mechanisms. For instance, arthritis biologics like etanercept and infliximab effectively target tumor necrosis factors—a feat still unattainable by small-molecule drugs. Additionally, compared to small-molecule drugs, large-molecule drugs exhibit fewer unexpected toxicities, which is a major reason biologics have replaced chemical drugs as the new favorites in the pharmaceutical market.
**Low Production Barriers: Small-Molecule Drugs Prone to Price Drops**
From a marketing perspective, small-molecule drugs with fixed chemical structures—such as aspirin (composed of 21 atoms) and acetaminophen (20 atoms)—are easily replicated. Once the original drug's patent expires, generic versions with identical components flood the market, causing prices to plummet.
In contrast, large-molecule drugs (biologics) have extremely high molecular weights and complex structures. For example, antibody drugs consist of around 15,000 atoms, making replication difficult.For this reason, some compare chemical drugs to bicycles, while biologic drugs are likened to jet planes—the complexity and expertise required for their production far surpass that of making a bicycle.
Consequently, replicating biologic drugs (commonly referred to as biosimilars) is even more challenging. Even biologics produced by the original manufacturers exhibit slight variations between batches, let alone biosimilars made by different companies. Regulating biosimilars presents a significant challenge for drug approval and regulatory authorities worldwide.
**How to Tell the Difference: Oral vs. Injection**
In this era of biologic drugs, do you know whether you are using small-molecule chemical drugs or large-molecule biologics? Are you taking an original biologic (Biological) or a biosimilar (Biosimilar)? Here’s a simple way to distinguish between chemical and biologic drugs: chemical drugs may come in oral, injectable, or even patch forms, whereas biologics are mostly administered via injection. This is because proteins or antibodies would be destroyed by stomach acid if taken orally, making injections the primary method of delivery.
As for whether you are using an original biologic (Biological) or a biosimilar (Biosimilar), patients have the right to know. You can consult your doctor or pharmacist for clarity and peace of mind regarding your medication.
Source: https://udn. com/ news/ story/ 7266/ 2570664
Top 10 Prescription Drug Rankings Shift as Biologics Gain Popularity


Xu Zu'an: Researcher at the Institute of Biotechnology and Pharmaceutical Research, National Health Research Institutes
The global top 10 prescription drug rankings are quietly undergoing a transformation. In 2006, the list of top-selling prescription drugs was dominated by small-molecule drugs (chemical drugs), with only two large-molecule drugs (biologics) making it into the top 10. However, a decade later in 2016, the rankings saw a dramatic reversal, with biologics—especially antibodies and protein-based drugs—rising in popularity, while chemical drugs barely held onto the list, with only three remaining.
**High Toxicity and Unpredictability: Small-Molecule Drugs Hit Roadblocks**
Why has the market share of biologics and chemical drugs undergone such a drastic shift? Whether protein-based drugs or monoclonal antibodies, they are broadly categorized as large-molecule drugs, distinct from chemically synthesized drugs (small-molecule drugs) with clearly defined molecular structures. Over the past decade, large-molecule drugs have gone from niche to mainstream, and this shift can be examined from two perspectives.
From a research and development standpoint, small-molecule drugs (chemical drugs) tend to have higher toxicity. The most troublesome issue in small-molecule drug development is the unpredictable toxicological reactions that arise when the drug or its metabolites enter the human body, often leading to safety-related roadblocks and development failures. On the other hand, advancements in pharmaceutical technology have allowed large-molecule drugs—including hormones, proteins, and antibody-based drugs—to overcome production barriers, leading to continuous innovation and gradual dominance in the market.
**Bionic Replacement Over Confrontation: The Rise of Large-Molecule Drugs**
Another critical reason for the widespread clinical application of biologics is the shift in medical thinking. In the past, treatment approaches were confrontational, such as aggressively eliminating cancer cells. However, in recent years, researchers have reconsidered disease treatment through a "return to nature" mechanism. This "bionics" concept—mimicking the natural functioning of biological systems—has introduced new perspectives for diseases facing treatment bottlenecks. Biologics (large-molecule drugs) are developed by emulating the body's natural regulatory mechanisms, allowing them to overcome the limitations of traditional small-molecule drugs in targeting certain diseases while offering more stable efficacy and safety.
Take insulin, the earliest biologic, as an example. Insulin was discovered a century ago as a treatment for diabetes, but it wasn’t until recombinant protein technology matured that it could be mass-produced to benefit more patients. Before that, insulin extracted from pigs was used, which often triggered immune rejection in humans. Antibodies, a crucial part of the body’s defense system, have also become a hot topic in new drug development due to advancements in antibody production technology.
For diseases involving immune dysregulation, such as cancer and autoimmune disorders, biologics activate the body's natural regulatory mechanisms. For instance, arthritis biologics like etanercept and infliximab effectively target tumor necrosis factors—a feat still unattainable by small-molecule drugs. Additionally, compared to small-molecule drugs, large-molecule drugs exhibit fewer unexpected toxicities, which is a major reason biologics have replaced chemical drugs as the new favorites in the pharmaceutical market.

**Low Production Barriers: Small-Molecule Drugs Prone to Price Drops**
From a marketing perspective, small-molecule drugs with fixed chemical structures—such as aspirin (composed of 21 atoms) and acetaminophen (20 atoms)—are easily replicated. Once the original drug's patent expires, generic versions with identical components flood the market, causing prices to plummet.
In contrast, large-molecule drugs (biologics) have extremely high molecular weights and complex structures. For example, antibody drugs consist of around 15,000 atoms, making replication difficult.For this reason, some compare chemical drugs to bicycles, while biologic drugs are likened to jet planes—the complexity and expertise required for their production far surpass that of making a bicycle.
Consequently, replicating biologic drugs (commonly referred to as biosimilars) is even more challenging. Even biologics produced by the original manufacturers exhibit slight variations between batches, let alone biosimilars made by different companies. Regulating biosimilars presents a significant challenge for drug approval and regulatory authorities worldwide.
**How to Tell the Difference: Oral vs. Injection**
In this era of biologic drugs, do you know whether you are using small-molecule chemical drugs or large-molecule biologics? Are you taking an original biologic (Biological) or a biosimilar (Biosimilar)? Here’s a simple way to distinguish between chemical and biologic drugs: chemical drugs may come in oral, injectable, or even patch forms, whereas biologics are mostly administered via injection. This is because proteins or antibodies would be destroyed by stomach acid if taken orally, making injections the primary method of delivery.
As for whether you are using an original biologic (Biological) or a biosimilar (Biosimilar), patients have the right to know. You can consult your doctor or pharmacist for clarity and peace of mind regarding your medication.
Source: https://udn. com/ news/ story/ 7266/ 2570664

